Graphene and batteries
Graphene, a sheet of carbon atoms bound together in a honeycomb lattice pattern, is hugely recognized as a “wonder material†due to the myriad of astonishing attributes it holds. It is a potent conductor of electrical and thermal energy, extremely lightweight chemically inert, and flexible with a large surface area. It is also considered eco-friendly and sustainable, with unlimited possibilities for numerous applications.
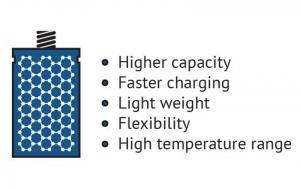 The advantages of graphene batteries
The advantages of graphene batteries
In the field of batteries, conventional battery electrode materials (and prospective ones) are significantly improved when enhanced with graphene. A graphene battery can be light, durable and suitable for high capacity energy storage, as well as shorten charging times. It will extend the battery’s life, which is negatively linked to the amount of carbon that is coated on the material or added to electrodes to achieve conductivity, and graphene adds conductivity without requiring the amounts of carbon that are used in conventional batteries.
Graphene can improve such battery attributes as energy density and form in various ways. Li-ion batteries (and other types of rechargeable batteries) can be enhanced by introducing graphene to the battery’s anode and capitalizing on the material’s conductivity and large surface area traits to achieve morphological optimization and performance.
It has also been discovered that creating hybrid materials can also be useful for achieving battery enhancement. A hybrid of Vanadium Oxide (VO2) and graphene, for example, can be used on Li-ion cathodes and grant quick charge and discharge as well as large charge cycle durability. In this case, VO2 offers high energy capacity but poor electrical conductivity, which can be solved by using graphene as a sort of a structural “backbone†on which to attach VO2 - creating a hybrid material that has both heightened capacity and excellent conductivity.
Another example is LFP (Lithium Iron Phosphate) batteries, that is a kind of rechargeable Li-ion battery. It has a lower energy density than other Li-ion batteries but a higher power density (an indicator of of the rate at which energy can be supplied by the battery). Enhancing LFP cathodes with graphene allowed the batteries to be lightweight, charge much faster than Li-ion batteries and have a greater capacity than conventional LFP batteries.
In addition to revolutionizing the battery market, combined use of graphene batteries and graphene supercapacitors could yield amazing results, like the noted concept of improving the electric car’s driving range and efficiency. While graphene batteries have not yet reached widespread commercialization, battery breakthroughs are being reported around the world.
Battery basics
Batteries serve as a mobile source of power, allowing electricity-operated devices to work without being directly plugged into an outlet. While many types of batteries exist, the basic concept by which they function remains similar: one or more electrochemical cells convert stored chemical energy into electrical energy. A battery is usually made of a metal or plastic casing, containing a positive terminal (an anode), a negative terminal (a cathode) and electrolytes that allow ions to move between them. A separator (a permeable polymeric membrane) creates a barrier between the anode and cathode to prevent electrical short circuits while also allowing the transport of ionic charge carriers that are needed to close the circuit during the passage of current. Finally, a collector is used to conduct the charge outside the battery, through the connected device.
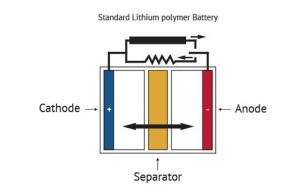
When the circuit between the two terminals is completed, the battery produces electricity through a series of reactions. The anode experiences an oxidation reaction in which two or more ions from the electrolyte combine with the anode to produce a compound, releasing electrons. At the same time, the cathode goes through a reduction reaction in which the cathode substance, ions and free electrons combine into compounds. Simply put, the anode reaction produces electrons while the reaction in the cathode absorbs them and from that process electricity is produced. The battery will continue to produce electricity until electrodes run out of necessary substance for creation of reactions.
Battery types and characteristics
Batteries are divided into two main types: primary and secondary. Primary batteries (disposable), are used once and rendered useless as the electrode materials in them irreversibly change during charging. Common examples are the zinc-carbon battery as well as the alkaline battery used in toys, flashlights and a multitude of portable devices. Secondary batteries (rechargeable), can be discharged and recharged multiple times as the original composition of the electrodes is able to regain functionality. Examples include lead-acid batteries used in vehicles and lithium-ion batteries used for portable electronics.
Batteries come in various shapes and sizes for countless different purposes. Different kinds of batteries display varied advantages and disadvantages. Nickel-Cadmium (NiCd) batteries are relatively low in energy density and are used where long life, high discharge rate and economical price are key. They can be found in video cameras and power tools, among other uses. NiCd batteries contain toxic metals and are environmentally unfriendly. Nickel-Metal hydride batteries have a higher energy density than NiCd ones, but also a shorter cycle-life. Applications include mobile phones and laptops. Lead-Acid batteries are heavy and play an important role in large power applications, where weight is not of the essence but economic price is. They are prevalent in uses like hospital equipment and emergency lighting.
Lithium-Ion (Li-ion) batteries are used where high-energy and minimal weight are important, but the technology is fragile and a protection circuit is required to assure safety. Applications include cell phones and various kinds of computers. Lithium Ion Polymer (Li-ion polymer) batteries are mostly found in mobile phones. They are lightweight and enjoy a slimmer form than that of Li-ion batteries. They are also usually safer and have longer lives. However, they seem to be less prevalent since Li-ion batteries are cheaper to manufacture and have higher energy density.
Batteries and supercapacitors
While there are certain types of batteries that are able to store a large amount of energy, they are very large, heavy and release energy slowly. Capacitors, on the other hand, are able to charge and discharge quickly but hold much less energy than a battery. The use of graphene in this area, though, presents exciting new possibilities for energy storage, with high charge and discharge rates and even economical affordability. Graphene-improved performance thereby blurs the conventional line of distinction between supercapacitors and batteries.
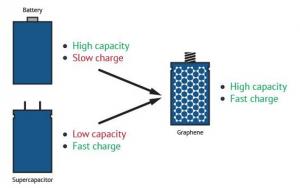 Graphene batteries combine the advantages of both batteries and supercapacitors
Graphene batteries combine the advantages of both batteries and supercapacitors
Graphene-enhanced batteries are almost here
Graphene-based batteries have exciting potential and while they are not yet fully commercially available yet, R&D is intensive and will hopefully yield results in the future. Companies all over the world (including Samsung, Huawei, and others) are developing different types of graphene-enhanced batteries, some of which are now entering the market. The main applications are in electric vehicles and mobile devices.
Some batteries use graphene in peripheral ways - not in the battery chemistry. For example in 2016, Huawei unveiled a new graphene-enhanced Li-Ion battery that uses graphene to remain functional at higher temperature (60° degrees as opposed to the existing 50° limit) and offer a double the operation time. Graphene is used in this battery for better heat dissipation - it reduces battery's operating temperature by 5 degrees.
Further reading
- Introduction to graphene
- Graphene Supercapacitors
- How to invest in the graphene revolution
- The Graphene Handbook, our very own guide to the graphene market
- Graphene-Info's graphene batteries market report
- Graphene supercapacitors market report
The latest graphene batteries news:
Honeycomb Battery Company and Nubia Brand International announce closing of business combination
Last year, Global Graphene Group (G3) announced plans for its subsidiary, Honeycomb Battery, to merge with a SPAC company (Nubia Brand International Corp.) in a deal worth $925 million. Earlier this month, it was announced that this business combination was completed.
Upon the completion of the business combination, the combined company was renamed Solidion Technology. Beginning on Monday, February 5, 2024, Solidion’s common stock was expected to begin trading on the NASDAQ Global Market under the new ticker symbol “STI.”
GMG updates on progress of its graphene-aluminum batteries
Graphene Manufacturing Group (GMG) has provided a progress update on its Graphene Aluminum-Ion Battery technology being developed by GMG and the University of Queensland (UQ).
The Company has announced it has produced multiple battery pouch cells with over 1000 mAh (1 Ah) capacity. In a recent build to confirm repeatability, the Company's development team has built and confirmed multiple cells, all reportedly testing greater than 1Ah (1000mAh). This is defined by GMG as "a major milestone achieved to demonstrate scalability from coin cells to pouch cells".
Lyten secures $4 Million grant from Department of Energy
Lyten has announced it has secured a $4 million grant from the U.S. Department of Energy (DoE) to accelerate the manufacturing of its advanced lithium-sulfur battery technology. This grant (awarded by the DoE’s Energy Efficiency and Renewable Energy / Vehicle Technologies Office) specifically targets lithium-sulfur technologies that can alleviate offshore supply chain risk for EV batteries and increase EV driving range.
Lithium-sulfur is a chemistry known for decades to potentially hold 2 to 3 times the energy density of lithium-ion but was not envisioned to come into the market until the 2030s due to material science challenges. Lyten set out to accelerate this timeline by using its 3D Graphene material to develop a sulfur-graphene composite cathode. In June 2023, Lyten opened a semi-automated lithium-sulfur pilot line producing pouch and cylindrical cells on its 145,000-square-foot campus in Silicon Valley and will begin to deliver non-EV cells commercially in 2024.
India-based Ipower Batteries launches graphene series lead-acid batteries
According to a recent announcement, India-based IPower Batteries has launched graphene series lead-acid batteries.
The company has claimed its new battery variants have been tested by ICAT for AIS0156 and have been awarded the Type Approval Certificate TAC for their innovative graphene series lead-acid technology.
Nanotech Energy announces its graphene-based batteries are available for pre-order
It was recently announced that Nanotech Energy is offering its graphene-based 18650 cells for pre-order through its commercialization partner, Voltaplex.
The cells bring together Nanotech Energy's electrolyte and proprietary electrodes with Soteria metallized polymer current collectors to create a major advancement in battery technology. The result, as per the Company, is a 100% American-made non-flammable lithium-ion battery pack that has shown its strength and flexibility survived a remarkable abuse trial.
Nanotech Energy announces launch of graphene batteries production plant Chico 2
Nanotech Energy has announced that graphene-based battery cells will go into full production in early 2024 at its new 150MW manufacturing facility Chico 2.
Nanotech Energy successfully completed trial weeks at Chico 2 in November and December. Almost all equipment is now in place at the Chico, CA site, and final processes are being refined ahead of a launch that will eventually generate 30,000 18650 battery cells per day.
Directa Plus enters deal for system to be used for graphene compounds
Directa Plus has revealed that it has signed a deal with an unnamed Italian innovator to buy the technology for a system capable of preparing tailored graphene compounds. According to the Company, this technology will initially be used in batteries and polymers.
It explained that the acquired know-how and technology would help to enable the dry encapsulation of G Plus graphene nanoplatelets into different compound carriers for different exacting applications.
NanoXplore announces commissioning of graphene-enhanced silicon and anode active material pilot lines
NanoXplore has announced the successful commissioning of two anode material pilot lines, reportedly achieving remarkable energy density and product validation. The Company called this 'a pivotal moment in NanoXplore’s ongoing commitment to advancing sustainable energy storage solutions'.
NanoXplore’s proprietary silicon graphene technology (SiG™) produced in the pilot line, has achieved an energy density of 1150 Wh/L, and demonstrated compatibility with conventional graphite anodes, resulting in an energy density of over 800 Wh/L. NanoXplore remains dedicated to pushing the boundaries of energy storage capabilities using its advanced materials technology. The SiG™ family is supported by 11 patents which cover a range of different chemistries and extend to all cylindrical cell form factors.
Nanotech Energy in search of new US brownfield gigafactory site
U.S-based graphene batteries developer Nanotech Energy has stated its intention to secure a new US brownfield gigafactory site early in 2024 that will accelerate its commercialization of graphene-based non-flammable lithium-ion batteries.
The announcement follows Nanotech Energy's successful sale of its Reno, NV, site and represents the Company's ambition to satisfy increased customer demand. It plans to use the site for an up to 6GWh-per-year plant, marking the next step in its journey to bring US-made fast charging, high energy density and inherently safe batteries to market. The Company hopes the new gigafactory will be operational in 2025.
Researchers develop antimony alloy based reduced graphene oxide composite for faster charging sodium-ion batteries
Researchers from the University of Hyderabad (UoH) and the Tata Institute of Fundamental Research (TIFR) have developed electrode materials made of Tin antimony alloy based reduced graphene oxide composite which has the potential to enhance energy storage for sodium-ion batteries.
Sodium-ion batteries could offer enhanced energy efficiency, rapid charging capabilities, resilience to extreme temperatures, and safeguards against overheating or thermal runaway incidents. They exhibit reduced toxicity due to their lack of reliance on lithium, cobalt, copper, or nickel, which have the potential to emit environmentally harmful gases in the event of fire, according to a recent official release.
Pagination
- Previous page
- Page 2
- Next page

