Researchers from Brown University designed the world's best non-platinum catalyst, based on cobalt-graphene. This can be used to replace Platinum with a more durable and less expensive material as a fuel-cell catalyst.
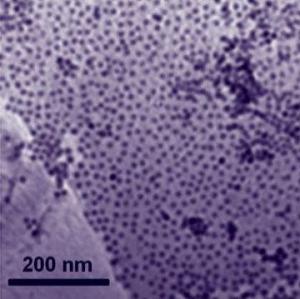
To create this new material, the researchers used a self-assembly method. First, they dispersed cobalt nanoparticles and graphene in separate solutions. The two solutions were then combined and pounded with sound waves to make sure they mixed thoroughly. That caused the nanoparticles to attach evenly to the graphene in a single layer. Using a centrifuge, the material was removed from the solution, and it was then dried. Exposing it to air, the outside layers of atomic cobalt on each nanoparticle are oxidized which forms a shell of cobalt-oxide that helps protect the cobalt core.