Researchers at Stanford University in California have developed a new material comprising interspersed layers of graphene and phosphorene that has been shown to be a more stable, more conductive and higher capacity anode for sodium ion batteries than previous materials. The researchers believe it could be industrially compatible, and potentially allow sodium ion batteries to become useful for large-scale energy storage.
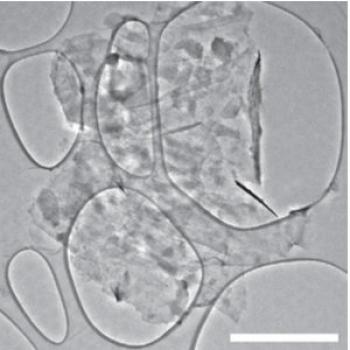
The graphene layers provide an elastic buffer and function as an electrical highway, allowing charge to get in and out faster. The phosphorene and graphene were both produced by scalable liquid exfoliation, and the sandwich structure self-assembled when suspensions of the two components were mixed and the solvent was evaporated.
The scientists are now working to improve this further and say that if they will be able to increase the life to 3000 cycles, the technology could be "very attractive for large-scale stationary energy storage" of electricity in the national grid. A possible hindrance might be awaiting the researchers on the way to improving the cycling performance, though. It is established that electrolytes tend to be thermodynamically unstable during intercalation in alkaline ion batteries. Li-ion anodes rapidly form a 'passivation layer' that sticks to the anode and protects the electrolyte, but this partially breaks down in Na-ion batteries every time the battery is discharged. Therefore, there is much more side reaction than in the case of the lithium. Scientists add that the high surface area of graphene could even make the problem worse.

