University of Illinois team finds that defects in graphene membranes may improve biomolecule transport
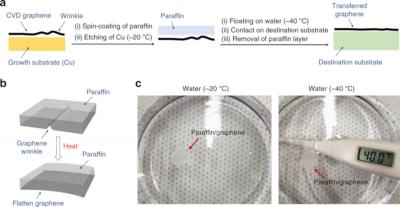
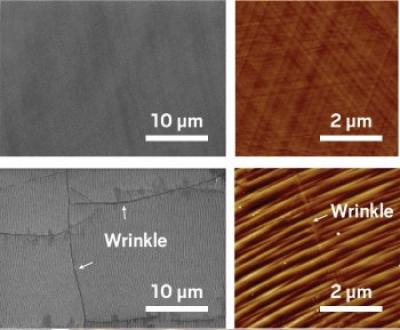
Researchers at the University of Illinois examined how tiny defects in graphene membranes, formed during fabrication, could be used to improve molecule transport. They found that the defects make a big difference in how molecules move along a membrane surface. Instead of trying to fix these flaws, the team set out to use them to help direct molecules into the membrane pores.
Nanopore membranes have generated interest in biomedical research because they help researchers investigate individual molecules - atom by atom - by pulling them through pores for physical and chemical characterization. This technology could ultimately lead to devices that can quickly sequence DNA, RNA or proteins.