Graphene to significantly improve gold catalyst for fuel cells
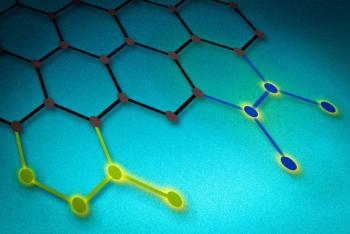
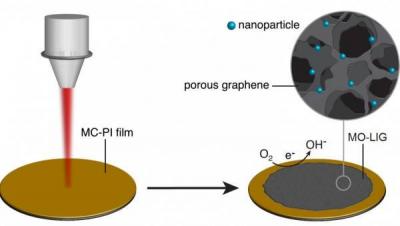
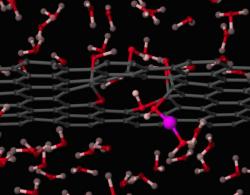
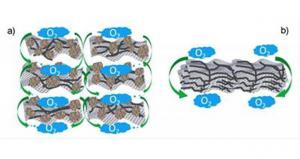
A team of researchers at Kyushu University's International Institute for Carbon-Neutral Energy Research (I2CNER) designed a method that could prove a real breakthrough technology for fuel cells - by showing how wrapping a graphene support in a specially prepared polymer provides an excellent foundation for making uniform, highly active gold nanoparticle catalysts.
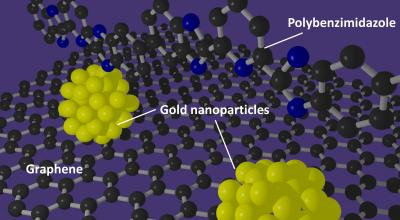
Gold nanoparticles have been recognized as an agreeable solution for improving the performance of catalysts that could be used in fuel cells, but creating a uniform, useful catalyst still remained elusive. However, the team in this study devised a method for using a new type of catalyst support. By wrapping the support in the polymer that was developed, the scientists created a much better support environment for the gold nanoparticles.