An international team of scientists has designed a molecule that uses light or electricity to convert the greenhouse gas carbon dioxide into carbon monoxide. The process includes using a nanographene-rhenium complex connected via an organic compound known as bipyridine to trigger a highly efficient reaction that could someday replace solar cells.
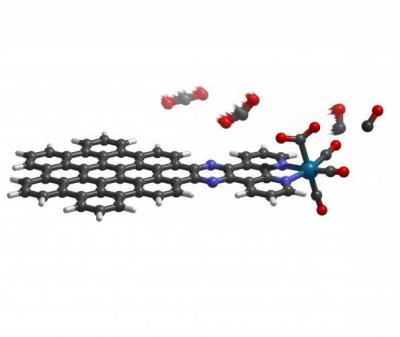
The molecule acts as a two-part system: a nanographene "energy collector" that absorbs energy from sunlight and an atomic rhenium "engine" that produces carbon monoxide. The energy collector drives a flow of electrons to the rhenium atom, which repeatedly binds and converts the normally stable carbon dioxide to carbon monoxide. The idea to link nanographene to the metal arose from earlier efforts to create a more efficient solar cell with the carbon-based material. But this model actually eliminates the solar cells, and uses the light-absorbing quality of nanographene alone to drive the reaction.
"If you can create an efficient enough molecule for this reaction, it will produce energy that is free and storable in the form of fuels," said an associate professor in the IU Bloomington College of Arts and Sciences. "This study is a major leap in that direction. The ability to efficiently and exclusively create carbon monoxide is significant due to the molecule's versatility. This is also a way to store energy as a carbon-neutral fuel since you're not putting any more carbon back into the atmosphere than you already removed. You're simply re-releasing the solar power you used to make it."
The bipyridine-metal complexes have long been studied to reduce carbon dioxide to carbon monoxide with sunlight but these molecules can use only a tiny sliver of the light in sunlight, primarily in the ultraviolet range, which is invisible to the naked eye. In contrast, the molecule developed in this study takes advantage of the light-absorption nanographene to create a reaction that uses sunlight in the wavelength up to 600 nm, a larger portion of the visible light spectrum.
The team plans to make the molecule more powerful, including making it last longer and survive in a non-liquid form as solid catalysts are easier to use in the real world. The scientists are also working to replace the rhenium atom in the molecule (a rare element) with manganese, a more common and less expensive metal.



