Graphene batteries: Introduction and Market News
Graphene and batteries
Graphene, a sheet of carbon atoms bound together in a honeycomb lattice pattern, is hugely recognized as a wonder material due to the myriad of astonishing attributes it holds. It is a potent conductor of electrical and thermal energy, extremely lightweight chemically inert, and flexible with a large surface area. It is also considered eco-friendly and sustainable, with unlimited possibilities for numerous applications.
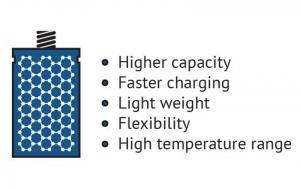
The advantages of graphene batteries
In the field of batteries, conventional battery electrode materials (and prospective ones) are significantly improved when enhanced with graphene. A graphene battery can be light, durable and suitable for high capacity energy storage, as well as shorten charging times. It will extend the battery's life, which is negatively linked to the amount of carbon that is coated on the material or added to electrodes to achieve conductivity, and graphene adds conductivity without requiring the amounts of carbon that are used in conventional batteries.
Graphene can improve such battery attributes as energy density and form in various ways. Li-ion batteries (and other types of rechargeable batteries) can be enhanced by introducing graphene to the battery's anode and capitalizing on the material's conductivity and large surface area traits to achieve morphological optimization and performance.
It has also been discovered that creating hybrid materials can also be useful for achieving battery enhancement. A hybrid of Vanadium Oxide (VO2) and graphene, for example, can be used on Li-ion cathodes and grant quick charge and discharge as well as large charge cycle durability. In this case, VO2 offers high energy capacity but poor electrical conductivity, which can be solved by using graphene as a sort of a structural backbone on which to attach VO2 - creating a hybrid material that has both heightened capacity and excellent conductivity.
Another example is LFP (Lithium Iron Phosphate) batteries, that is a kind of rechargeable Li-ion battery. It has a lower energy density than other Li-ion batteries but a higher power density (an indicator of of the rate at which energy can be supplied by the battery). Enhancing LFP cathodes with graphene allowed the batteries to be lightweight, charge much faster than Li-ion batteries and have a greater capacity than conventional LFP batteries.
In addition to revolutionizing the battery market, combined use of graphene batteries and graphene supercapacitors could yield amazing results, like the noted concept of improving the electric car's driving range and efficiency. While graphene batteries have not yet reached widespread commercialization, battery breakthroughs are being reported around the world.
Battery basics
Batteries serve as a mobile source of power, allowing electricity-operated devices to work without being directly plugged into an outlet. While many types of batteries exist, the basic concept by which they function remains similar: one or more electrochemical cells convert stored chemical energy into electrical energy. A battery is usually made of a metal or plastic casing, containing a positive terminal (an anode), a negative terminal (a cathode) and electrolytes that allow ions to move between them. A separator (a permeable polymeric membrane) creates a barrier between the anode and cathode to prevent electrical short circuits while also allowing the transport of ionic charge carriers that are needed to close the circuit during the passage of current. Finally, a collector is used to conduct the charge outside the battery, through the connected device.
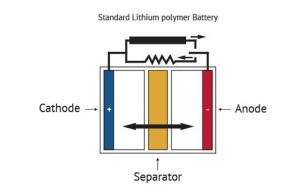
When the circuit between the two terminals is completed, the battery produces electricity through a series of reactions. The anode experiences an oxidation reaction in which two or more ions from the electrolyte combine with the anode to produce a compound, releasing electrons. At the same time, the cathode goes through a reduction reaction in which the cathode substance, ions and free electrons combine into compounds. Simply put, the anode reaction produces electrons while the reaction in the cathode absorbs them and from that process electricity is produced. The battery will continue to produce electricity until electrodes run out of necessary substance for creation of reactions.
Battery types and characteristics
Batteries are divided into two main types: primary and secondary. Primary batteries (disposable), are used once and rendered useless as the electrode materials in them irreversibly change during charging. Common examples are the zinc-carbon battery as well as the alkaline battery used in toys, flashlights and a multitude of portable devices. Secondary batteries (rechargeable), can be discharged and recharged multiple times as the original composition of the electrodes is able to regain functionality. Examples include lead-acid batteries used in vehicles and lithium-ion batteries used for portable electronics.
Batteries come in various shapes and sizes for countless different purposes. Different kinds of batteries display varied advantages and disadvantages. Nickel-Cadmium (NiCd) batteries are relatively low in energy density and are used where long life, high discharge rate and economical price are key. They can be found in video cameras and power tools, among other uses. NiCd batteries contain toxic metals and are environmentally unfriendly. Nickel-Metal hydride batteries have a higher energy density than NiCd ones, but also a shorter cycle-life. Applications include mobile phones and laptops. Lead-Acid batteries are heavy and play an important role in large power applications, where weight is not of the essence but economic price is. They are prevalent in uses like hospital equipment and emergency lighting.
Lithium-Ion (Li-ion) batteries are used where high-energy and minimal weight are important, but the technology is fragile and a protection circuit is required to assure safety. Applications include cell phones and various kinds of computers. Lithium Ion Polymer (Li-ion polymer) batteries are mostly found in mobile phones. They are lightweight and enjoy a slimmer form than that of Li-ion batteries. They are also usually safer and have longer lives. However, they seem to be less prevalent since Li-ion batteries are cheaper to manufacture and have higher energy density.
Batteries and supercapacitors
While there are certain types of batteries that are able to store a large amount of energy, they are very large, heavy and release energy slowly. Capacitors, on the other hand, are able to charge and discharge quickly but hold much less energy than a battery. The use of graphene in this area, though, presents exciting new possibilities for energy storage, with high charge and discharge rates and even economical affordability. Graphene-improved performance thereby blurs the conventional line of distinction between supercapacitors and batteries.
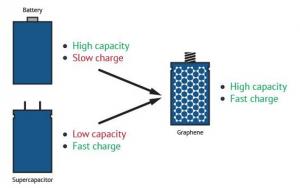 Graphene batteries combine the advantages of both batteries and supercapacitors
Graphene batteries combine the advantages of both batteries and supercapacitors
Graphene-enhanced batteries are almost here
Graphene-based batteries have exciting potential and while they are not yet fully commercially available yet, R&D is intensive and will hopefully yield results in the future. Companies all over the world (including Samsung, Huawei, and others) are developing different types of graphene-enhanced batteries, some of which are now entering the market. The main applications are in electric vehicles and mobile devices.
Some batteries use graphene in peripheral ways - not in the battery chemistry. For example in 2016, Huawei unveiled a new graphene-enhanced Li-Ion battery that uses graphene to remain functional at higher temperature (60° degrees as opposed to the existing 50° limit) and offer a double the operation time. Graphene is used in this battery for better heat dissipation - it reduces battery's operating temperature by 5 degrees.
Further reading
- Introduction to graphene
- Graphene Supercapacitors
- How to invest in the graphene revolution
- The Graphene Handbook, our very own guide to the graphene market
- Graphene-Info's graphene batteries market report
- Graphene supercapacitors market report
Graphene coating helps improve lithium-ion battery cathodes
Researchers at the California Institute of Technology (Caltech) have developed a method for coating lithium-ion battery cathodes with graphene, extending their life and performance. This recent effort may improve lithium-ion battery performance and reduce reliance on cobalt, an element frequently used in lithium-ion batteries that is difficult to source sustainably.
Image credit: Caltech
Caltech senior research scientist, David Boyd, has worked over the past decade to develop techniques for manufacturing graphene. In 2015, Boyd and colleagues discovered that high-quality graphene could be produced at room temperature. Prior to this, the production of graphene required extremely high temperatures, up to 1,000 degrees Celsius. After this breakthrough, they searched for new applications for graphene. Recently, Boyd teamed up with Will West, a technologist at Caltech's Jet Propulsion Laboratory (JPL), which Caltech manages for NASA. West specializes in electrochemistry and, in particular, in the development of improved battery technologies. Boyd and West set out to see if graphene could create an improved lithium-ion battery, which they have shown to be possible.
GMG launches a graphene solution for the lithium-ion battery industry
Graphene Manufacturing Group (GMG) has announced the launch of SUPER G®, a graphene slurry which can be used to enhance the performance of lithium-ion batteries. This product has, according to GMG, the potential to reshape the future of energy storage, offering battery manufacturers an innovative solution that optimizes efficiency, power, and longevity.

SUPER G® is a graphene slurry which has been developed by GMG over the last 3 years for GMG’s own Graphene Aluminum-Ion Battery which has unique properties of high electrical conductivity, low charge transfer resistance and high density compared to other carbon battery additives and materials used in lithium-ion batteries.
Solidion Technology updates on third quarter 2024 results
Solidion Technology, an advanced silicon anode and battery technology materials provider, has announced its operational and financial results for the third quarter of 2024. The Company declared a $4.2 million loss from continuing operations, including increased spending on third-party validation testing for automakers. Net Loss was $6,636,679.

Solidion Technology also mentioned technological and business developments. Among the technological advancements were: developing and securing a newly granted U.S. patent for technology enabling 5-minute charging of lithium batteries across all climates by leveraging a graphene-based heat spreader for optimal battery temperature control, expanding the Company's industry-leading intellectual property portfolio 1 with 20 new U.S. patents granted this year and achieving third-party validation for the Company's cost-effective process that eliminates the need for toxic silane gas and CVD techniques.
Volt Carbon and its subsidiary Solid UltraBattery awarded DAIR funding
Volt Carbon Technologies, in collaboration with Downsview Aerospace Innovation & Research (DAIR), has announced that both Volt Carbon's southern Ontario-based mineral processing operation (Toronto) and its subsidiary, Solid UltraBattery (Guelph), have been selected as recipients of the DAIR Green Fund.
The funded projects include: 1) "Development of High-Performance Carbon Materials for Aerospace Industries" and 2) "Advancements in Low-Temperature Performance of Lithium-Ion Batteries." These initiatives aim to advance battery solutions for unmanned aerial systems and enhance the capabilities of graphite and graphene in aerospace materials.
Lyten to acquire battery manufacturing assets from Northvolt
Lyten has announced that it is acquiring manufacturing assets from Northvolt, a Swedish battery manufacturer facing financial difficulties.
Northvolt is selling manufacturing equipment the company inherited in its 2021 acquisition of Cuberg, another battery startup. Lyten will also assume the lease of Cuberg’s old manufacturing facility in San Leandro, California. Lyten will invest $20 million next year to expand facilities in San Leandro and its existing operations in San Jose.
Project Arrow gets further funding, sports graphene-based battery by VoltaXplore
Project Arrow aims to develop an all-electric SUV concept with autonomous driving capabilities, and is a collaboration between nearly 60 different companies in Canada (led by the Automotive Parts Manufacturers’ Association (APMA)). In 2023, at the CES (Consumer Electronics Show) event in Las Vegas, a fully operational prototype was unveiled.

Project Arrow concept unveiled at CES
Now, the Canadian government announced a new investment of CAD$7 million (just over USD$5 million) in the project.
Graphene-Info updates its Graphene Batteries Market Report
Today we published a new edition of our Graphene Batteries Market Report, with all the latest information and updates from companies and researchers in the field. The batteries market is extremely active, as demand from EVs and mobile applications increases R&D efforts, and graphene is seen as a potential material to increase capacity, decrease charging times and improve other performance metrics.
Reading this report, you'll learn all about:
- The advantages of using graphene in batteries
- The different ways graphene can be used in batteries
- Various types of graphene materials
- What's on the market today
The report package also provides:
- A list of all graphene companies involved with batteries
- Detailed specifications of graphene-enhanced anode materials
- Personal contact details into most graphene developers
- Free updates for a year
This Graphene Batteries market report provides a great introduction to graphene materials used in the batteries market, and covers everything you need to know about graphene in this niche. This is a great guide for anyone involved with the battery market, nanomaterials, electric vehicles and mobile devices.
Solidion develops a graphene-enabled battery fast-charging and cooling system
Solidion Technology has announced that it has been granted a patent on a cost-effective graphene-based strategy for enabling completion of charging in 5 minutes for a wide range of lithium batteries.

Range anxiety, the fear that an electric vehicle (EV) may run out of battery power during a trip, has long been regarded as a key reason for consumers' reluctance to adopt EVs. This issue is exacerbated by the notion that recharging batteries usually takes much longer time than refueling internal combustion engine vehicles (ICEVs). To be competitive with ICEVs, fast charging of EVs should be weather-independent and comparable in time as refueling a gasoline car. Variations in temperatures in different geographic regions and seasons poses a challenge in fast charging EV batteries, since batteries can behave very differently. None of today's EV batteries allow for fast charging at low temperatures.
Komaki launches electric scooter that is said to use graphene battery
It was reported that Indian electric vehicle brand Komaki has introduced the new model of Cat 3.0 NXT that comes with two battery variants, Graphene and LIPO4, and will be available for Rs. 1,19,999 (around USD$1400) and Rs. 1,49,999 (almost USD$1800). The unveiling of this EV is aimed at last-mile delivery operators, enabling sustainable all-day use and supporting SMEs and MSMEs in growing their businesses.
The EV features app-based battery options, Graphene and LIPO4, giving a range of over 180 km to 200 km on a single charge, depending on the battery type.
Graphene Manufacturing Group engages with Australian government on battery solutions
Graphene Manufacturing Group (GMG) has provided an update on its ongoing engagement with the Australian Federal Government, highlighting recent meetings with key officials.
GMG’s leadership, including CEO Craig Nicol, met with Senator Tim Ayres, Assistant Minister for Trade, to discuss the Company’s battery manufacturing progress and how federal policies like the Future Made in Australia Battery Breakthrough could benefit GMG. Further discussions took place with Speaker of the House of Representatives Milton Dick and Queensland Senator Anthony Chisholm, building on prior visits to GMG's facilities in Richlands, Queensland.
Pagination
- Page 1
- Next page



