Graphene Supercapacitors: Introduction and News
Graphene supercapacitors
Graphene is a thin layer of pure carbon, tightly packed and bonded together in a hexagonal honeycomb lattice. It is widely regarded as a “wonder material†because it is endowed with an abundance of astonishing traits: it is the thinnest compound known to man at one atom thick, as well as the best known conductor. It also has amazing strength and light absorption traits and is even considered ecologically friendly and sustainable as carbon is widespread in nature and part of the human body.
Graphene is often suggested as a replacement for activated carbon in supercapacitors, in part due to its high relative surface area (which is even more substantial than that of activated carbon). The surface area is one of the limitations of capacitance and a higher surface area means a better electrostatic charge storage. In addition, graphene based supercapacitors will utilize its lightweight nature, elastic properties and mechanical strength.
A Graphene supercapacitor is said to store almost as much energy as alithium-ion battery, charge and discharge in seconds and maintain all this over tens of thousands of charging cycles. One of the ways to achieve this is by using a a highly porous form of graphene with a large internal surface area (made by packing graphene powder into a coin-shaped cell and then dry and press it).
What are supercapacitors?
Supercapacitors, also known as EDLC (electric double-layer capacitor) or Ultracapacitors, differ from regular capacitors in that they can store tremendous amounts of energy.
A basic capacitor usually consists of two metal plates, separated by an insulator (like air or a plastic film). During charging, electrons accumulate on one conductor and depart from the other. One side gains a negative charge while the other side builds a positive one. The insulator disturbs the natural pull of the negative charge towards the positive one, and that tension creates an electric field. Once electrons are given a path to the other side, discharge occurs.
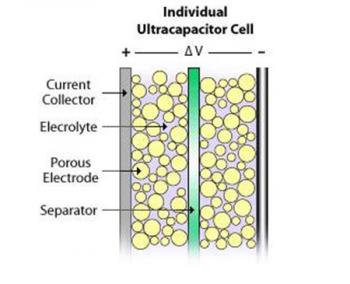
Supercapacitors also contain two metal plates, only coated with a porous material known as activated carbon. They are immersed in an electrolyte made of positive and negative ions dissolved in a solvent. One plate is positive and the other is negative. During charging, ions from the electrolyte accumulate on the surface of each carbon-coated plate. Supercapacitors also store energy in an electric field that is formed between two oppositely charged particles, only they have the electrolyte in which an equal number of positive and negative ions is uniformly dispersed. Thus, during charging, each electrode ends up having two layers of charge coating (electric double-layer).
Batteries and Supercapacitors
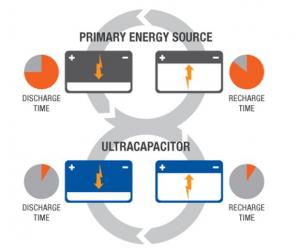
Unlike capacitors and supercapacitors, batteries store energy in a chemical reaction. This way, ions are inserted into the atomic structure of an electrode, instead of just clinging to it like in supercapacitors. This makes supercapacitors (and storing energy without chemical reactions in general) able to charge and discharge much faster than batteries. Due to the fact that a supercapacitor does not suffer the same wear and tear as a chemical reaction based battery, it can survive hundreds of thousands more charge and discharge cycles.
Supercapacitors boast a high energy storage capacity compared to regular capacitors, but they still lag behind batteries in that area. Supercapacitors are also usually more expensive per unit than batteries. Technically, it is possible to replace the battery of a cell phone with a supercapacitor, and it will charge much faster. Alas, it will not stay charged for long. Supercapacitors are very effective, however, at accepting or delivering a sudden surge of energy, which makes them a fitting partner for batteries. Primary energy sources such as internal combustion engines, fuel cells and batteries work well as a continuous source of low power, but cannot efficiently handle peak power demands or recapture energy because they discharge and recharge slowly. Supercapacitors deliver quick bursts of energy during peak power demands and then quickly store energy and capture excess power that's otherwise lost. In the example of an electric car, a supercapacitor can provide needed power for acceleration, while a battery provides range and recharges the supercapacitor between surges.
Common supercapacitor applications
Supercapacitors are currently used to harvest power from regenerative braking systems and release power to help hybrid buses accelerate, provide cranking power and voltage stabilization in start/stop systems, backup and peak power for automotive applications, assist in train acceleration, open aircraft doors in the event of power failures, help increase reliability and stability of the energy grid of blade pitch systems, capture energy and provide burst power to assist in lifting operations, provide energy to data centers between power failures and initiation of backup power systems, such as diesel generators or fuel cells and provide energy storage for firming the output of renewable installations and increasing grid stability.
Rivaling materials
Several materials exist that are researched and suggested to augment supercapacitors as much (or even more than) graphene. Among these materials are: hemp, that was used by Canadian researchers to develop hemp fibers that are at least as efficient as graphene ones in supercapacitor electrodes, Cigarette filters, which were used by Korean researchers to prepare a material for supercapacitor electrodes that exhibits a better rate capability and higher specific capacitance than conventional activated carbon and even higher than N-doped graphene or N-doped CNT electrodes.
Graphene supercapacitors commercialization
Graphene supercapacitors are already on the market, and several companies, including Skeleton Technology, the CRRC, ZapGoCharger, and Angstron Materials are developing such solutions. Read our Graphene Supercapacitors market report to learn more about this exciting market and how graphene will effect it.
Further reading
- Graphene-Info's graphene supercapacitors market report
- Graphene batteries
- Introduction to graphene
- How to invest in the graphene revolution
- The Graphene Handbook, our very own guide to the graphene market
NETL team converts coal tar pitch into graphene for improved supercapacitors
Researchers at NETL, a U.S. Department of Energy national laboratory, have developed a low-cost process for converting coal tar waste into high-quality graphene. The team stressed that the resulting can increase the performance of energy-storing supercapacitor systems by up to 55%.
Schematic illustration of the synthesis process of 3D graphene. Image from: Small Methods
According to NETL’s Christopher Matranga, one of the authors of the study, graphene has long been considered an ideal supercapacitor electrode material, but its use in commercial devices is limited because there are few methods for producing high-quality graphene at a large scale at a low cost.
Victoria government gives funding boost to graphene supercapacitors developer EnyGy
The Victorian government’s AUD$1 million (around USD$650,000) Made In Victoria Energy Technologies Manufacturing program will be awarded to four Victorian businesses manufacturing renewable energy technology components and products.

Aming recipients is graphene technologies company EnGy, which will use its grant to scale up graphene materials production.
Inkjet printed silver/graphene flexible composite electrodes enable high-performance supercapacitors
Researchers at the Technical University of Liberec (Czech Republic) and Lodz University of Technology (Poland) have developed a silver/graphene flexible composite electrode using inkjet printing technology for high-performance supercapacitors.
The scientists chose rGO as the primary material for the electrode active layer. The rGO active layer was in-situ printed and reduced on the polypropylene non-woven fabric, and silver nanoparticles were simultaneously inserted and reduced to increase the interlayer spacing of the rGO active layer, which effectively reduced the self-stacking effect of rGO and improved the overall electrochemical performance.
Graphene-Info publishes a new edition of its Graphene Supercapacitors Market Report
Today we published a new edition of our Graphene Supercapacitors Market Report, with all the latest information. The supercapacitor market and industry is facing high demand and graphene is a pivotal material for this application.
Reading this report, you'll learn all about:
- The advantages of using graphene in supercapacitors
- Various types of graphene materials
- Market insights and forecasts
- What's on the market today
The report package also provides:
- A list of all graphene companies involved with supercapacitors
- Prominent research activity in this field
- Free updates for a year
This Graphene Supercapacitors market report provides a great introduction to graphene materials used in the supercapacitor market, and covers everything you need to know about graphene in this niche. This is a great guide for anyone involved with the supercapacitor market, nanomaterials, electric vehicles and mobile devices.
A sustainable approach for graphene paste could enable microsupercapacitors and multipurpose flexible electronics
Researchers from Italy's Sapienza University of Rome, Portugal's International Iberian Nanotechnology Laboratory (INL), University of Minho and Universidade de Lisboa have developed a sustainable approach to produce an electrically conductive, graphene-based paste suitable for fabricating flexible devices such as microsupercapacitors (mSCs).
The new method enables the scalable, cost-effective, and environmentally friendly production of high-quality graphene materials, paving the way for advanced applications in energy storage and flexible electronics.
HydroGraph to supply graphene to Volfpack Energy for solar power battery storage
HydroGraph Clean Power has announced that its flagship graphene product, FGA-1, has been chosen by Volfpack Energy, a hardware company focused on using supercapacitor technology to increase the adoption of renewable energy across Asia.
Its flagship product, fractal graphene, FGA-1, was chosen by Volfpack to be the base material of the supercapacitor design after Volfpack’s engineers determined that it outperformed materials traditionally used in supercapacitors, such as activated carbon, by 4x.
Researchers develop deformable micro-supercapacitor via laser ablation patterning of Graphene/liquid metal
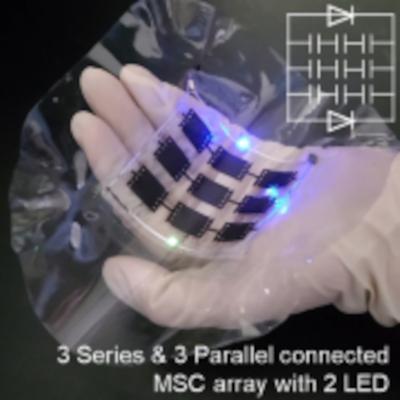
Researchers from Pohang University of Science and Technology (POSTECH), Korea Institute of Industrial Technology and Konkuk University have reported the development of a small-scale energy storage device capable of stretching, twisting, folding, and wrinkling.
Nine MSC units connected in three parallel and three series. Image from npj Flexible Electronics
Micro supercapacitors (MSCs) have emerged as a promising candidate for deformable energy storage, due to high-power density, rapid charging, and long cycle life. However, the fabrication of interdigitated electrode patterns capable of maintaining the energy storage performance under repeated stretching and twisting has remained a great challenge, because brittle materials like gold (Au) have been commonly used as an electrode.
Graphene-Info publishes a new edition of its Graphene Supercapacitors Market Report
Today we published a new edition of our Graphene Supercapacitors Market Report, with all the latest information. The supercapacitor market and industry is facing high demand and graphene is a pivotal material for this application. This new update includes many updates from various projects and research activities
Reading this report, you'll learn all about:
- The advantages of using graphene in supercapacitors
- Various types of graphene materials
- Market insights and forecasts
- What's on the market today
The report package also provides:
- A list of all graphene companies involved with supercapacitors
- Prominent research activity in this field
- Free updates for a year
This Graphene Supercapacitors market report provides a great introduction to graphene materials used in the supercapacitor market, and covers everything you need to know about graphene in this niche. This is a great guide for anyone involved with the supercapacitor market, nanomaterials, electric vehicles and mobile devices.
Researchers develop deformable graphene-based liquid metal micro-supercapacitors
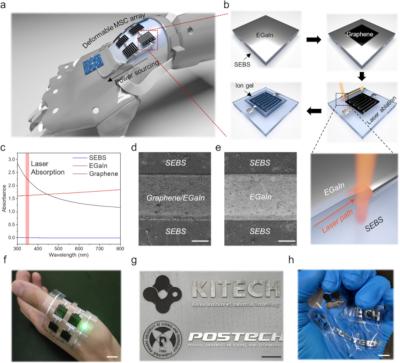
Researchers from Pohang University of Science and Technology (POSTECH), Korea Institute of Industrial Technology and Konkuk University have fabricated highly deformable graphene-based micro supercapacitors (MSCs) using liquid metal current collectors on an elastic polymer substrate.
a Illustration of an integrated system comprising soft-electronics and deformable energy storage component. b The fabrication process of EGaIn-based MSC. c UV-vis spectra of SEBS, EGaIn, and graphene. FE-SEM images of laser ablated d Graphene/EGaIn and e EGaIn (Scale bar = 200 µm). Photographs of f institute logos, g deformed logos, and h an LED connected to the MSC circuit (Scale bar = 1 cm). (Image from npj Flexible Electronics)
The team used eutectic gallium-indium (EGaIn), a liquid metal alloy, as the current collector since a deformable current collector is needed in order to create a deformable MSC. Commonly used current collectors made of brittle materials like gold (Au) are not suitable in this case, so the team turned to 'liquid metal' that inherently possesses the properties of a liquid and metallic conductivity.
Researchers develop improved graphene micro supercapacitors for wearables
Researchers from China University of Petroleum (East China), Henan Agricultural University and Chinese Academy of Sciences have developed an additive-free 3D printing process to construct graphene micro-supercapacitors (MSCs) with unprecedented electrochemical properties and seamless integrability. The team states that this achievement overcomes existing manufacturing limitations and brings closer the on-chip MSC arrays essential for the next generation of wearables.
Wearable devices require ever-smaller on-board energy solutions that can deliver bursts of power while remaining unobtrusive. Rigid coin batteries restrict device flexibility and ergonomics. Leading microscale alternatives include micro-supercapacitors (MSCs), which store and discharge energy rapidly owing to highly porous electrode materials interfacing with electrolytes. Supercapacitors’ quick charge ability and resilience to repeated charging cycles make them appealing to supplement batteries. However, difficulties producing intricately designed MSC devices that also offer high performance have confined MSCs to the lab. Conventional manufacturing techniques often lack suitable precision, flexibility, and scalability.
Pagination
- Page 1
- Next page