University of Cambridge researchers have designed a lithium-ion battery that can be directly charged in sunlight. This was done in an effort to improve the general process of connecting solar panels to batteries to store energy when the sun is shining.
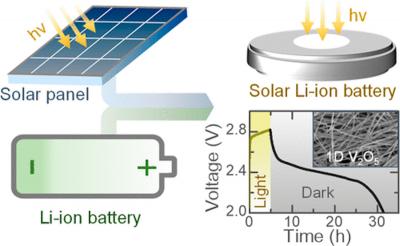
The idea is to simplify how solar energy is harvested and stored, says Michael De Volder, a mechanical engineer at the University of Cambridge who led the work. If the team can improve the efficiency and lifetime of the hybrid device, its cost will likely be lower than combining solar cells and batteries. For the price of a battery, you get both functionalities, he says.
The new light-rechargeable battery is based on a cathode made of vanadium pentoxide nanofibers. The material stores lithium ions and also harvests light to generate paired electrons and positive charges, or holes. The researchers mixed the nanofibers with poly(3-hexylthiophene-2,5-diyl) (P3HT) that blocks the movement of holes, and graphene oxide that aids electron transport.
To make the battery, they drilled a hole on the cathode side of a coin cell and placed a glass window in it to let light through. When the device is illuminated, electrons generated at the cathode move through an external circuit to the lithium anode. Meanwhile, the holes trigger the vanadium cathode to release lithium ions, which migrate through the electrolyte and combine with the electrons at the anode to form lithium, charging the battery.
Other teams have attempted to make light-rechargeable batteries, but they could only be recharged 10 times or so. The new device lasts for over 200 cycles. It’s also reportedly three times as efficient. Efficiency measures the amount of energy the battery can deliver compared with the solar energy it absorbs.
At 2.6%, the device’s efficiency is still too low for practical use. However, the team is starting to explore materials other than vanadium pentoxide for the photocathode and is thinking carefully about cathode design to push efficiencies higher.
More research on other parts of the battery such as electrolytes and the interfaces between the electrodes and electrolytes would also help, says Karim Zaghib, a materials engineer at McGill University. With improvements in efficiency, he says he can see a device like this becoming a sustainable power source for small sensors and consumer electronics devices.



