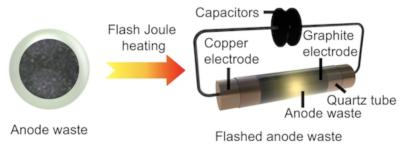
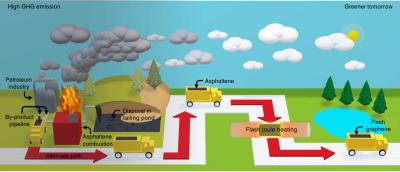
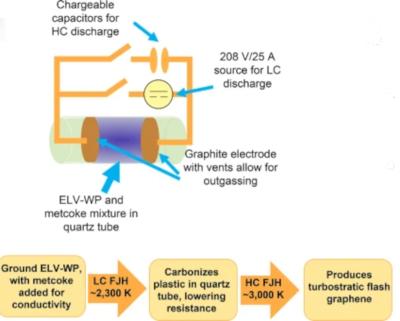
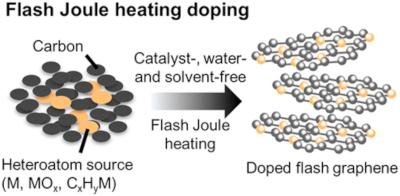
Rice researchers map the diffusion of graphene and hexagonal boron nitride in aqueous solutions
Rice University researchers have mapped out how bits of 2D materials move in liquid ⎯ which that could help scientists assemble macroscopic-scale materials with the same useful properties as their 2D counterparts.
In order to maintain these special properties in bulk form, sheets of 2D materials have to be properly aligned ⎯ a process that often occurs in solution phase. The Rice team focused on graphene and hexagonal boron nitride, a material with a similar structure to graphene but composed of boron and nitrogen atoms.