Researchers at the Harvey Mudd College in the U.S have made an unexpected discovery that holds exciting potential for creating robust water-repellent coatings using gas-phase-synthesized graphene (GSG) and other nanomaterials.
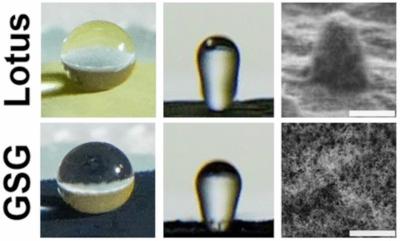
Last summer, the researchers were working on two projects: A National Science Foundation-funded project characterizing nanocomposites and a project supported by the College’s Rasmussen Summer Research fund that involved using graphene to separate oil from water. While working on these projects, the students discovered that water droplets falling on our graphene powder were easily sliding off and bouncing from it, says Albert Dato, author of the paper. This was a completely unexpected result since we weren’t even looking into this phenomenon called superhydrophobicity.
Miller and Parkinson discovered that the graphene produced in the Dato Lab can repel water immediately after it is created. Graphene that is grown by other methods cannot achieve such water repellency without considerable chemical modification, and graphene that is made from exfoliating graphite also needs to be treated with chemicals to be able to repel water. Our method of making graphene is sustainable and produces useful byproducts that can be collected and utilized in other applications, says Dato. In contrast, creating graphene by other methods requires significant energy and resources, and chemical modification can produce hazardous waste that is harmful to the environment.
The team decided to continue the research during the academic year, focusing on investigating what would happen if they coated other materialsâmetals, silicon, Scotch tapeâwith their graphene. Although we discovered something novel in the summer, we wanted to see if we could take surfaces that were not water repellent and coat them with our graphene to make the surfaces non-wettable, Dato says.
With the goal of testing the hypothesis that their graphene could protect surfaces from water droplets, Miller and Parkinson conducted experiments that showed exactly that: arbitrary wettable surfaces became non-wettable when covered with their graphene. We decided to report our discovery to as wide of an audience as soon as possible, Dato says.
In the paper, we share some preliminary results of GSG on Scotch tape, but we want to test stronger adhesives to make more robust coatings, which is essential for actually using a material’s superhydrophobic properties in the real world, says Parkinson. One of the next things we’re focusing on is creating robust superhydrophobic coatings. We’re also looking at other carbon nanomaterials, since we now have established procedures and have the equipment necessary for contact angle measurements, roll-off angle measurements and droplet impact studies.
That, Dato says, is exciting because highly water repellent surfaces are desired in numerous applications, such as aircraft wings that resist icing in cold weather, ships that can resist corrosion and have low drag forces and even biomedical devices that can have tiny channels that can transport fluids with minimal effort. The graphene produced in our lab can potentially make these applications a reality.

