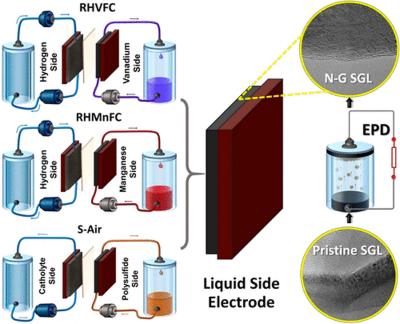
Following a Global Business innovation Programme initiated in 2019, and a collaborative visit to the US, the UK established a new Graphene Innovation Group (UK-GIG) that puts together 15 specialists from UK companies that together share many years of experience in graphene and expertise across the entire value chain.

Scott Storey, a Business Innovation Advisor at Inventya and the lead coordinator at the UK-GIG, explains more about the group - "We can take an everyday industrial challenge, apply our combined graphene knowledge, engineer and manufacture an effective solution, and take that solution to national and international markets. UK-GIG is now an established cohort of 15 UK-based graphene companies, ranging from early stage through to established businesses. The UK-GIG companies are fully aware of the huge potential for graphene technology to improve materials or be used in novel ways across multiple sectors. They are seeking collaboration opportunities where they can combine their expertise to do what they do best - solve problems, design graphene applications, and help commercialize an increasingly exciting field. They aim to make the UK the best place on earth to be grafting in graphene!"
We have reached out to some of the GIG members, to find out how has graphene effected their business and products, and the effects of graphene on their materials or devices.