G6 Materials and Gilman Industries start work on graphene-based green energy project
 G6 Materials Corp. (formerly known as "Graphene 3D Lab) has announced the start of a new green-energy focused collaboration with Gilman Industries, a company focused on commercializing its hydrogen-producing technology. The objective of the project is to develop a new generation of Evolve, a proprietary hydrogen generator that produces hydrogen by splitting water with an electric current.
G6 Materials Corp. (formerly known as "Graphene 3D Lab) has announced the start of a new green-energy focused collaboration with Gilman Industries, a company focused on commercializing its hydrogen-producing technology. The objective of the project is to develop a new generation of Evolve, a proprietary hydrogen generator that produces hydrogen by splitting water with an electric current.
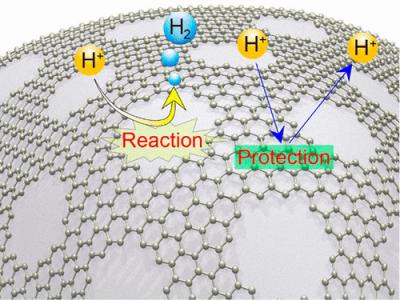
During the course of this project, G6 will develop a robust graphene-based material for electrodes within the hydrogen generator. Introducing a resilient graphene-based material has the potential to deliver chemical stability that could allow the generator to operate with seawater, which if successful, would drastically expand the range of potential applications.