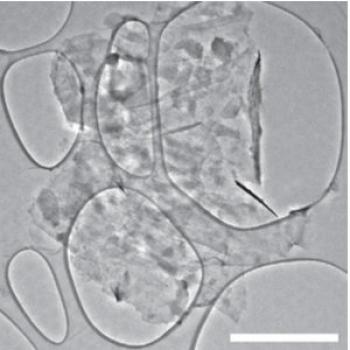
Graphene "cages" may open the door to silicon Li-ion battery anodes
A team of scientists at Stanford University and the Department of Energy's SLAC National Accelerator Laboratory has come up with a possible answer to the question of how to make lithium-ion battery anodes out of silicon, as these tend to swell and crack, as well as react with the battery electrolyte to form a coating that harms their performance.
The scientists wrapped each silicon anode particle in a custom-fit "cage" made of graphene, in a simple, three-step method for building microscopic graphene cages of just the right size: roomy enough to let the silicon particle expand as the battery charges, yet tight enough to hold all the pieces together when the particle falls apart, so it can continue to function at high capacity. The strong, flexible cages also block destructive chemical reactions with the electrolyte.