Graphene NEMS switch for electrostatic discharge protection applications
Researches from the University of California, Riverside and University of California, Los Angeles have demonstrated a novel above-IC graphene NEMS switches for electrostatic discharge (ESD) protection applications.
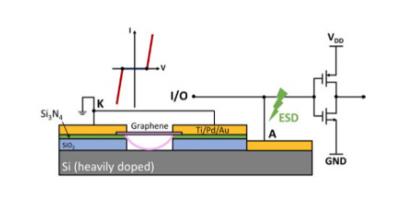
This graphene ESD switch is a two-terminal device with a gap between the conducting substrate at the bottom and a suspended graphene membrane on top serving as the discharging path. This new concept provides a potentially revolutionary mechanism for the on-chip ESD protections.