Graphene and batteries
Graphene, a sheet of carbon atoms bound together in a honeycomb lattice pattern, is hugely recognized as a wonder material due to the myriad of astonishing attributes it holds. It is a potent conductor of electrical and thermal energy, extremely lightweight chemically inert, and flexible with a large surface area. It is also considered eco-friendly and sustainable, with unlimited possibilities for numerous applications.
The advantages of graphene batteries
In the field of batteries, conventional battery electrode materials (and prospective ones) are significantly improved when enhanced with graphene. A graphene battery can be light, durable and suitable for high capacity energy storage, as well as shorten charging times. It will extend the battery's life, which is negatively linked to the amount of carbon that is coated on the material or added to electrodes to achieve conductivity, and graphene adds conductivity without requiring the amounts of carbon that are used in conventional batteries.
Graphene can improve such battery attributes as energy density and form in various ways. Li-ion batteries (and other types of rechargeable batteries) can be enhanced by introducing graphene to the battery's anode and capitalizing on the material's conductivity and large surface area traits to achieve morphological optimization and performance.
It has also been discovered that creating hybrid materials can also be useful for achieving battery enhancement. A hybrid of Vanadium Oxide (VO2) and graphene, for example, can be used on Li-ion cathodes and grant quick charge and discharge as well as large charge cycle durability. In this case, VO2 offers high energy capacity but poor electrical conductivity, which can be solved by using graphene as a sort of a structural backbone on which to attach VO2 - creating a hybrid material that has both heightened capacity and excellent conductivity.
Another example is LFP (Lithium Iron Phosphate) batteries, that is a kind of rechargeable Li-ion battery. It has a lower energy density than other Li-ion batteries but a higher power density (an indicator of of the rate at which energy can be supplied by the battery). Enhancing LFP cathodes with graphene allowed the batteries to be lightweight, charge much faster than Li-ion batteries and have a greater capacity than conventional LFP batteries.
In addition to revolutionizing the battery market, combined use of graphene batteries and graphene supercapacitors could yield amazing results, like the noted concept of improving the electric car's driving range and efficiency. While graphene batteries have not yet reached widespread commercialization, battery breakthroughs are being reported around the world.
Battery basics
Batteries serve as a mobile source of power, allowing electricity-operated devices to work without being directly plugged into an outlet. While many types of batteries exist, the basic concept by which they function remains similar: one or more electrochemical cells convert stored chemical energy into electrical energy. A battery is usually made of a metal or plastic casing, containing a positive terminal (an anode), a negative terminal (a cathode) and electrolytes that allow ions to move between them. A separator (a permeable polymeric membrane) creates a barrier between the anode and cathode to prevent electrical short circuits while also allowing the transport of ionic charge carriers that are needed to close the circuit during the passage of current. Finally, a collector is used to conduct the charge outside the battery, through the connected device.
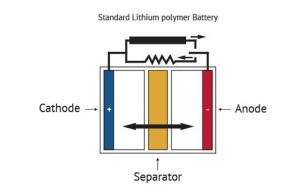
When the circuit between the two terminals is completed, the battery produces electricity through a series of reactions. The anode experiences an oxidation reaction in which two or more ions from the electrolyte combine with the anode to produce a compound, releasing electrons. At the same time, the cathode goes through a reduction reaction in which the cathode substance, ions and free electrons combine into compounds. Simply put, the anode reaction produces electrons while the reaction in the cathode absorbs them and from that process electricity is produced. The battery will continue to produce electricity until electrodes run out of necessary substance for creation of reactions.
Battery types and characteristics
Batteries are divided into two main types: primary and secondary. Primary batteries (disposable), are used once and rendered useless as the electrode materials in them irreversibly change during charging. Common examples are the zinc-carbon battery as well as the alkaline battery used in toys, flashlights and a multitude of portable devices. Secondary batteries (rechargeable), can be discharged and recharged multiple times as the original composition of the electrodes is able to regain functionality. Examples include lead-acid batteries used in vehicles and lithium-ion batteries used for portable electronics.
Batteries come in various shapes and sizes for countless different purposes. Different kinds of batteries display varied advantages and disadvantages. Nickel-Cadmium (NiCd) batteries are relatively low in energy density and are used where long life, high discharge rate and economical price are key. They can be found in video cameras and power tools, among other uses. NiCd batteries contain toxic metals and are environmentally unfriendly. Nickel-Metal hydride batteries have a higher energy density than NiCd ones, but also a shorter cycle-life. Applications include mobile phones and laptops. Lead-Acid batteries are heavy and play an important role in large power applications, where weight is not of the essence but economic price is. They are prevalent in uses like hospital equipment and emergency lighting.
Lithium-Ion (Li-ion) batteries are used where high-energy and minimal weight are important, but the technology is fragile and a protection circuit is required to assure safety. Applications include cell phones and various kinds of computers. Lithium Ion Polymer (Li-ion polymer) batteries are mostly found in mobile phones. They are lightweight and enjoy a slimmer form than that of Li-ion batteries. They are also usually safer and have longer lives. However, they seem to be less prevalent since Li-ion batteries are cheaper to manufacture and have higher energy density.
Batteries and supercapacitors
While there are certain types of batteries that are able to store a large amount of energy, they are very large, heavy and release energy slowly. Capacitors, on the other hand, are able to charge and discharge quickly but hold much less energy than a battery. The use of graphene in this area, though, presents exciting new possibilities for energy storage, with high charge and discharge rates and even economical affordability. Graphene-improved performance thereby blurs the conventional line of distinction between supercapacitors and batteries.
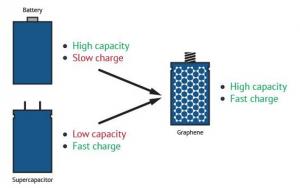 Graphene batteries combine the advantages of both batteries and supercapacitors
Graphene batteries combine the advantages of both batteries and supercapacitors
Graphene-enhanced batteries are almost here
Graphene-based batteries have exciting potential and while they are not yet fully commercially available yet, R&D is intensive and will hopefully yield results in the future. Companies all over the world (including Samsung, Huawei, and others) are developing different types of graphene-enhanced batteries, some of which are now entering the market. The main applications are in electric vehicles and mobile devices.
Some batteries use graphene in peripheral ways - not in the battery chemistry. For example in 2016, Huawei unveiled a new graphene-enhanced Li-Ion battery that uses graphene to remain functional at higher temperature (60° degrees as opposed to the existing 50° limit) and offer a double the operation time. Graphene is used in this battery for better heat dissipation - it reduces battery's operating temperature by 5 degrees.
Further reading
- Introduction to graphene
- Graphene Supercapacitors
- How to invest in the graphene revolution
- The Graphene Handbook, our very own guide to the graphene market
- Graphene-Info's graphene batteries market report
- Graphene supercapacitors market report
NanoGraf begins production at scale to provide Military with better batteries
NanoGraf, an advanced battery material company, announced earlier this month the successful completion of the first large volume production run of its M38 18650 cell for the U.S. military.
Nanograf, formerly called SiNode Systems, pursues advances in Lithium-ion battery anodes for a wide range of industries from consumer electronics to electric vehicles. NanoGraf explaines that it uses a proprietary silicon alloy-graphene material architecture to overcome the silicon anode technical hurdles. The combination of silicon-based alloys and a flexible 3D graphene network helps stabilize the active material during charge and discharge. The company’s manufacturing process is different from others that rely on expensive and complex vapor deposition-based systems. Instead, a wet chemistry process has been developed that is highly scalable and already proven in a multi-ton-scale pilot manufacturing line in Japan. The anode material drops into existing electrode mixing and coating equipment and has been validated in large-scale battery manufacturing facilities.
Luxembourg Future Fund 2 announces equity investment into Lyten
Lyten and the Luxembourg Future Fund 2 (LFF2) have announced that LFF2 has made an equity investment into Lyten.
The investment in Lyten follows the signing of a Memorandum of Understanding (MOU) in October 2023 to establish Lyten’s European headquarters in Luxembourg. The investment highlights Luxembourg’s commitment to advancing clean technologies and incorporating them into the European economy. Through this partnership, Lyten and Luxembourg will collaborate on research and development and the introduction of several Lyten products into the European market including its lithium-sulfur EV battery.
SalgenX announces process that turns dendrites into high-performance cathode materials
Salgenx, developer of graphene-based salt water flow battery technology, has announced the development of a method to transform dendrites into a key component for high-performance cathode metallic materials in the saltwater flow battery.
This new approach aims to not only mitigate the problems caused by dendrites but to also leverage their unique properties to enhance battery efficiency and longevity.
Indian EV startup iVOOMi launches electric scooter with graphene battery
An India-based EV startup called iVOOMi has launched the S1 Lite, an electric scooter available with two battery options, Graphene and Li-ion.
The graphene unit offers a range of up to 75 km and takes 7-8 hours to charge fully, while the Li-ion pack provides a range of up to 85 km and can be charged in under 4 hours. Both variants feature a 1.2 kW motor with 1.8 kW peak power and 10.1 Nm of torque.
Solidion plans to expand production capacity of silicon-rich graphene composite materials
Solidion Technology, an advanced battery technology solutions provider, has announced its plan to begin expanding the production capacity of silicon-rich graphene composite materials in early 2025.
The amount of energy that a lithium-ion battery can supply to an electric vehicle (EV) is limited by the amount of charges stored in its anode and cathode materials. Although graphite has been the preferred anode material during the past 30 years, silicon oxide (SiOx) and silicon (Si) are two evolving anode materials capable of improving the energy density of EV batteries and extending the EV range by 20-40%. However, the higher-capacity gain of both silicon and silicon oxide is limited by the technical issue of large volume change-induced rapid capacity decay and processing difficulty. Solidion has targeted this technical obstacle and has established a Dayton, Ohio-based facility for manufacturing silicon oxide (SiOx) and silicon (Si). The Solidion team is ready to expand the production capacity for these two types of high-capacity anode materials.
Graphene-Info updates its Graphene Batteries Market Report
Today we published a new edition of our Graphene Batteries Market Report, with all the latest information and updates from companies and researchers in the field. The batteries market is extremely active, as demand from EVs and mobile applications increases R&D efforts, and graphene is seen as a potential material to increase capacity, decrease charging times and improve other performance metrics.
Reading this report, you'll learn all about:
- The advantages of using graphene in batteries
- The different ways graphene can be used in batteries
- Various types of graphene materials
- What's on the market today
The report package also provides:
- A list of all graphene companies involved with batteries
- Detailed specifications of graphene-enhanced anode materials
- Personal contact details into most graphene developers
- Free updates for a year
This Graphene Batteries market report provides a great introduction to graphene materials used in the batteries market, and covers everything you need to know about graphene in this niche. This is a great guide for anyone involved with the battery market, nanomaterials, electric vehicles and mobile devices.
Researchers design graphene-based thermal regulator that enable safer lithium-ion batteries
Researchers at Tsinghua University, Zhejiang University and Zhejiang Sanhua Intelligent Controls Co., have designed a graphene-based thermal-switching material for improving the safety of lithium-ion batteries (LIBs) by making sure that they can safely operate at different temperatures and do not explode when overheated.
a) Thermal-switching mechanism of the TSM. b) The self-assembly process through freeze-casting of 2D-flake–microsphere suspensions to form an alternating multilayer scaffold together with polymer infiltration. Image credit: Nature Energy
A general approach to improving the safety of LIBs is using thermal-conducting interlayers, materials designed to even out the temperature between a battery's modules, bringing it to between 15 to 45 °C. To ensure that a high-capacity LIB is safe, these materials should be highly thermally insulating, thus preventing the propagation of heat, while also ensuring that temperature is uniformly distributed in the battery. The research team's newly developed thermal-switching material meets both criteria, and can effectively regulate the temperature in high-capacity batteries. This material rapidly responds to temperature, enabling the safe cycling of batteries in varying operating conditions.
Lyten ships Li-S battery A-samples for customer evaluation
Lyten has announced it has shipped A samples of its 6.5 Ah (C/3 discharge rate, 25° C) lithium-sulfur pouch cells to Stellantis and other leading US and EU automotive OEMs for evaluation. Lyten is known for using Li-S cathode made of sulfur and its proprietary 3D Graphene, sourced by capturing carbon from methane. This is said to eliminate the need for critical minerals like nickel, cobalt, and manganese in the cathode. The Li-S anode is a lithium metal composite, eliminating the need for graphite. The Company explaines that elimination of critical minerals means a projected 65%+ lower carbon footprint than lithium-ion batteries and a supply chain that can be fully sourced in the US or EU at scale.
Lyten manufactures lithium-sulfur cells in both pouch and cylindrical formats (2170 and 18650) and is currently shipping the 6.5 Ah pouch cell format for customer evaluation. Later this year, Lyten plans to deliver cylindrical A samples for evaluation. Lyten’s lithium-sulfur format flexibility enables its use in a wide range of industries beyond automotive, including space, aerospace, drones, micromobility, defense and consumer electronics.
Researchers use self-organized crack-free nanocellular graphene film to enhance sodium ion batteries
Researchers from Tohoku University, Tianjin University of Technology, Pohang University of Science and Technology and Johns Hopkins University recently designed a nanocellular graphene (NCG) film through the self-organization of carbon atoms using liquid metal dealloying and employing a defect-free amorphous precursor.
The flexible freestanding nanocellular graphene film. Image credit: Advanced Materials
Nanocellular graphene is a specialized form of graphene that achieves a large specific surface area by stacking multiple layers of graphene and controlling its internal structure with a nanoscale cellular morphology. NCG is attractive thanks to its potential to improve the performance of electronic devices, energy devices and sensors. However, its development has been hindered by defects that occur during the manufacturing process. Cracks often appear when forming NCG, and scientists are looking for new processing technologies that can fabricate homogeneous, crack-free and seamless NCGs at appropriate scales.
GMG secures funding to build graphene aluminum ion battery pilot plant
Graphene Manufacturing Group (GMG) has secured Queensland government backing for a proposed automated battery pilot plant for the manufacture of GMG’s Graphene Aluminum Ion Battery. The Company signed a Queensland Critical Minerals and Battery Technology Fund Agreement with the state for a grant of AUD$2 million (almost USD$1,300,000).
GMG is using graphene to produce aluminium-ion batteries utilizing a patent-pending surface perforation technology developed by the University of Queensland. GMG said the grant was for the payment of 50 percent of the capital cost of GMG’s proposed pilot plant, up to a maximum of $2 million.
Pagination
- Page 1
- Next page









