Rice University scientists have attached ruthenium atoms to graphene to create a durable catalyst for high-performance fuel cells. Most catalysts used to drive the oxygen reduction reaction that lets fuel cells turn chemical energy into electricity are made of platinum, which stands up to the acidic nature of the cell’s charge-carrying electrolyte. However, platinum is expensive, and replacements have long been searched for by researchers.
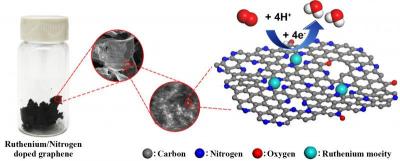
The ruthenium-graphene combination may pose a suitable replacement; In tests, its performance was said to easily match that of traditional platinum-based alloys and bested iron and nitrogen-doped graphene, another contender.
Spreading single ruthenium atoms across a sheet of graphene was reported as fairly straightforward. It involved dispersing graphene oxide in a solution, loading in a small amount of ruthenium and then freeze-drying the new solution and turning it into a foam. Baking that at 750 degrees Celsius (1,382 degrees Fahrenheit) in the presence of nitrogen and hydrogen gas reduced the graphene and locked nitrogen atoms to the surface, providing sites where ruthenium atoms could bind.
Materials made at higher and lower temperatures weren’t as good, and those made at the proper temperature but without either ruthenium or nitrogen proved the quality of the reaction depended on the presence of both.
The material reportedly showed excellent tolerance against methanol crossover and carbon monoxide poisoning in an acidic medium, both of which degrade the efficiency of fuel cells; such degradation is a persistent problem with traditional platinum fuel cells.



