An international team of scientists has designed a new way to produce single-layer graphene from a simple precursor: ethene (also known as ethylene) - the smallest alkene molecule, which contains just two atoms of carbon.
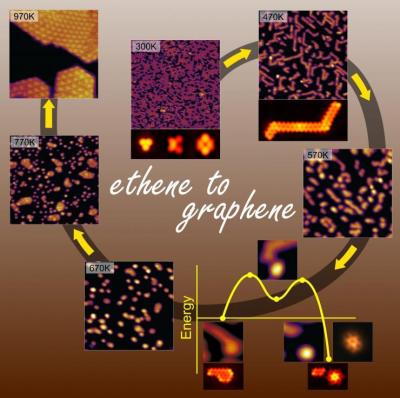
Heating the ethene to a temperature of slightly more than 700 degrees Celsius, the researchers reportedly produced pure layers of graphene on a rhodium catalyst substrate. The gradual heating and higher temperature overcame challenges that came up in earlier efforts to produce graphene directly from hydrocarbon precursors.
Guided by a theoretical approach, the researchers reasoned that the path from ethene to graphene would involve formation of a series of structures as hydrogen atoms leave the ethene molecules and carbon atoms self-assemble into the honeycomb pattern that characterizes graphene. To explore the nature of the thermally-induced rhodium surface-catalyzed transformations from ethene to graphene, the researchers raised the temperature of the material in steps under ultra-high vacuum. They used scanning-tunneling microscopy (STM), thermal programed desorption (TPD) and high-resolution electron energy loss (vibrational) spectroscopy (HREELS) to observe and characterize the structures that form at each step of the process.
The team explained that upon heating, ethene adsorbed onto the rhodium catalyst evolves via coupling reactions to form segmented one-dimensional polyaromatic hydrocarbons (1D-PAH). Further heating leads to dimensionality crossover one dimensional to two dimensional structures and dynamical restructuring processes at the PAH chain ends with a subsequent activated detachment of size-selective carbon clusters, following a mechanism revealed through first-principles quantum mechanical simulations. Finally, rate-limiting diffusional coalescence of these dynamically self-evolved cluster-precursors leads to condensation into graphene with high purity.
At the final stage before the formation of graphene, the researchers observed nearly round disk-like clusters containing 24 carbon atoms, which spread out to form the graphene lattice. The temperature must be raised within windows of temperature ranges to allow the requisite structures to form before the next stage of heating, the team explained. If you stop at certain temperatures, you are likely to end up with coking.
According to the researchers, an important component is the dehydrogenation process which frees the carbon atoms to form intermediate shapes, but some of the hydrogen resides temporarily on, or near, the metal catalyst surface and it assists in subsequent bond-breaking process that lead to detachment of the 24-carbon cluster-precursors. All along the way, there is a loss of hydrogen from the clusters. Bringing up the temperature essentially ‘boils’ the hydrogen out of the evolving metal-supported carbon structure, culminating in graphene.
The resulting graphene structure is adsorbed onto the catalyst. It may be useful attached to the metal, but for other applications, a way to remove it will have to be developed.
Thanks to its relative low cost and simplicity, the technique could be attractive for various applications. The research was conducted by scientists at the Georgia Institute of Technology, Technische Universität München in Germany, and the University of St. Andrews in Scotland. In the United States, the research was supported by the U.S. Air Force Office of Scientific Research and the U.S. Department of Energy’s Office of Basic Energy Sciences.