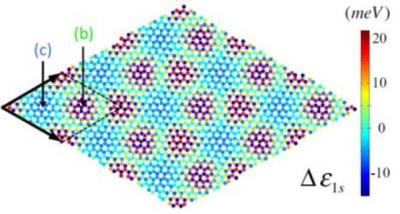
Researchers develop new Bernal-stacked bilayer graphene rapid production method
Researchers from the University of California and Rice University developed a new rapid method to synthesize high quality large-area Bernal (AB) stacked bilayer graphene sheets.
During the same research, the first bi-layer graphene double-gate field-effect transistor (G-FET) was also demonstrated. This G-FET features the best on/off switching ratio and carrier mobility.