Research develop new non-toxic method for producing high-quality graphene oxide
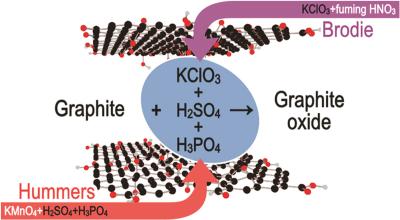
Researchers at Sweden's Umeå University, Lund University and Denmark's Aarhus University have reported a new way to synthesize graphene oxide, which has significantly fewer defects compared to materials produced by the most common method. To date, graphene oxide of similarly good quality could only be synthesized by using a rather dangerous method involving extremely toxic fuming nitric acid.
Graphene oxide is often used to produce graphene by removing oxygen. However, if there are holes in graphene oxide, there will also be holes after it is converted to graphene. Therefore, the quality of the graphene oxide is very important. Umeå University's Alexandr Talyzin and his research group have now addressed the issue of how to safely make good graphene oxide.