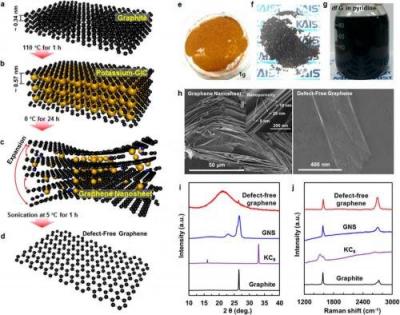
Researchers at Queen's University develop a novel, scalable and low-cost process to produce defect-free graphene nanoplatelets
Researchers at Queen’s University in Kingston, Canada have developed a simple yet effective exfoliation process for producing few-layer graphene nanoplatelets (FL-GNPs). Utilizing this one-step, chemical and solvent-free process the researchers were able to convert graphite flakes (+100 mesh, purity >97%) into FL-GNPs at a high yield (90%) and to subsequently form thermoplastic/FL-GNPs composites with improved electrical and mechanical properties.
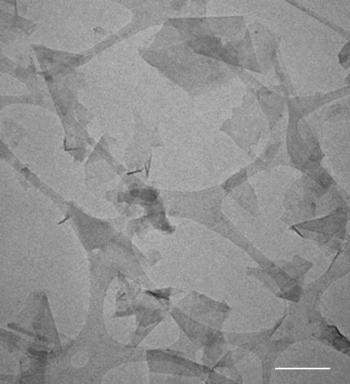
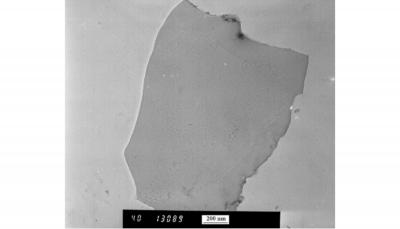 TEM image of isolated FL-GNP
TEM image of isolated FL-GNP
The exfoliated graphene nanoplatelets had a high specific surface area (325 m2/g), an aspect ratio above 500 (approximate lateral dimensions of 2µm and thickness of 3.5 nm), and a Raman D/G ratio of 0.3; indicating a structure with few defects. The flexural modulus of polyamide/FL-GNP composites containing 15 volume % FL-GNPs improved from 1850 MPa to 5,080 MPa while the electrical conductivity rose from 5x10-14 S/m to 21 S/m. Surface-coating the FL-GNPs through the addition of a coating agent during the last stages of the exfoliation process rendered the FL-GNPs more hydrophilic, thus, forming stable dispersions in water.