Graphene allows passage of protons, which could prove valuable for clean energy uses
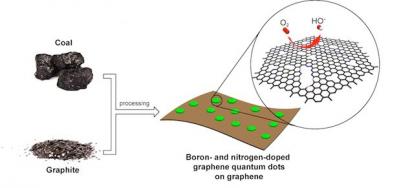
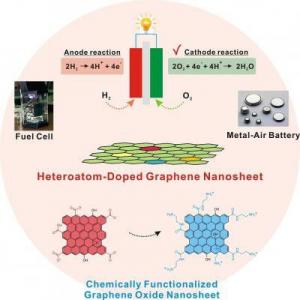
Researchers led by Prof. Andre Geim discovered that graphene, impermeable to gases and liquids, allows protons to pass. This is a breakthrough discovery that could make graphene suitable for use as a proton-conducting membrane, essential in fuel cell technology.
The scientists were surprised to find out that protons manage to pass through graphene with relative ease, especially in high temperatures, because graphene usually demonstrates barrier-like qualities. Fuel cells use oxygen and hydrogen as fuel and convert the input chemical energy unto electricity, with a significant issue of fuel leaks across traditional proton membranes (thus reducing efficiency). The scientists claim graphene membranes may fix that problem and make create much more efficient fuel cells.