Graphene enables the ideal chemical sensor
Researchers from the University of Illinois at Chicago (UIC) developed a graphene-based highly sensitive chemical sensor (an electronic "nose" if you will). The graphene enabled the researchers to increase the sensitivity to absorbed gas molecules by 300 times compared to current technology.

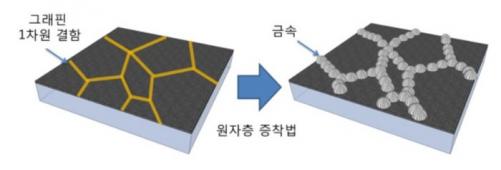
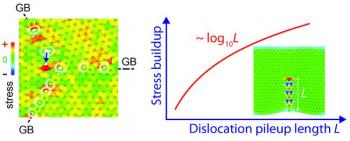
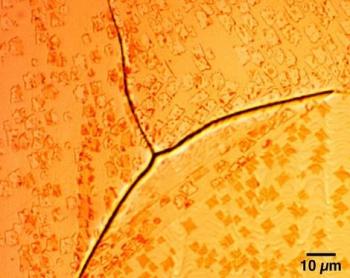
Interestingly, the graphene's grain boundaries are key to this achievement. The researchers discovered that the gas molecules are attracted to these grain boundaries, and so this is the ideal spot for the detection of these molecules. They explain that the irregular nature of the grain boundary produces hundreds of electron-transport gaps with different sensitivities - as if there are multiple switches all working in parallel.