Researchers from the University of Manchester have found that incorporating graphene oxide into three-dimensional scaffolds that support regenerating cartilage could offer a new means of delivering vital growth factors.
Damage to cartilage from injury or disease is difficult to remedy because of the material’s low capacity for self-repair. Future treatments hope to harness tissue-engineering approaches, introducing hydrogel scaffolds impregnated with stem cells that can proliferate and differentiate into chondrocytes, to make new cartilage. This strategy requires the appropriate biological cues to drive cell differentiation, but the results of various attempts to achieve sustained delivery of such signals have been disappointing.
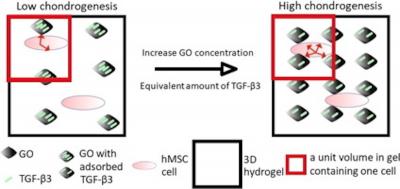
Now, for the first time, a team of researchers led by Judith A. Hoyland has incorporated transforming growth factor beta-3 (TGF-beta 3) absorbed onto flakes of graphene oxide (GO) into a collagen hydrogel. GO can absorb a wide variety of biological molecules onto its surface and has great potential as a carrier of active agents. The team added human mesenchymal stem cells (hMSCs) into the hydrogel and observed the results over 28 days in culture.
A modified Hummers’ method was used to synthesize the GO flakes, which ranged in size from 10 to 40 microns and just a few atomic layers thick. The GO flakes were simply mixed with a solution containing the growth factor and incubated for an hour. To create the scaffolds, the GO/ TGF-beta 3 solutions were added to pH 7 collagen solution, along with hMSCs taken from the bone marrow of patients’ with osteoarthritis.
The researchers believe that their approach has a number of advantages. The preparation of the hydrogel containing growth-factor-loaded GO is simple and straightforward. Meanwhile, the large surface area of GO means that only small quantities are needed to equal a similar amount of growth factor supplied externally. The ability of GO to retain TGF-beta 3 means that a slow release rate can be maintained over an extended time period. Moreover, the GO itself appears to be nontoxic to hMSCs.
The new approach provides and efficient growth factor delivery system, particularly in a 3D cell-encapsulated scaffold, with the potential to deliver multiple factors simultaneously, state the researchers. More generally, the potential of GO to deliver biological cues locally is an attractive strategy worth further exploration for tissue engineering, particularly for regionally-specific MSC differentiation.