Researchers from Rice University have transformed wood into an electrical conductor by turning its surface into graphene. The team used its LIG technique to blacken a thin film pattern onto a block of pine.

Previous work with LIG included heating the surface of a sheet of polyimide, an inexpensive plastic, with a laser. Rather than a flat sheet of hexagonal carbon atoms, LIG is a foam of graphene sheets with one edge attached to the underlying surface and chemically active edges exposed to the air.
The researchers said turning wood into graphene opens new avenues for the synthesis of LIG from nonpolyimide materials. "For some applications, such as three-dimensional graphene printing, polyimide may not be an ideal substrate," they said. "In addition, wood is abundant and renewable."
As with polyimide, the process takes place with a standard industrial laser at room temperature and pressure and in an inert argon or hydrogen atmosphere. Without oxygen, heat from the laser doesn't burn the pine but transforms the surface into wrinkled flakes of graphene foam bound to the wood surface. Changing the laser power also changed the chemical composition and thermal stability of the resulting LIG. At 70% power, the laser reportedly produced the highest quality of what they named "P-LIG," where the P stands for "pine."
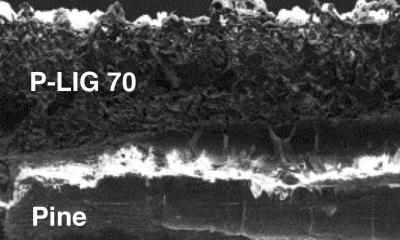
The lab took its discovery a step further by turning P-LIG into electrodes for splitting water into hydrogen and oxygen and supercapacitors for energy storage. For the former, they deposited layers of cobalt and phosphorus or nickel and iron onto P-LIG to make a pair of electrocatalysts with high surface areas that proved to be durable and effective.
Depositing polyaniline onto P-LIG turned it into an energy-storing supercapacitor that had usable performance metrics, Prof. James Tour said.
The scientists envision additional applications for the P-LIG, in such areas as solar energy for photosynthesis. Tour, however, saw a more immediate environmental benefit from biodegradable electronics: "Graphene is a thin sheet of a naturally occurring mineral, graphite, so we would be sending it back to the ground from which it came along with the wood platform instead of to a landfill full of electronics parts."



