Graphene and batteries
Graphene, a sheet of carbon atoms bound together in a honeycomb lattice pattern, is hugely recognized as a “wonder material†due to the myriad of astonishing attributes it holds. It is a potent conductor of electrical and thermal energy, extremely lightweight chemically inert, and flexible with a large surface area. It is also considered eco-friendly and sustainable, with unlimited possibilities for numerous applications.
 The advantages of graphene batteries
The advantages of graphene batteries
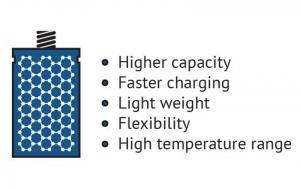
In the field of batteries, conventional battery electrode materials (and prospective ones) are significantly improved when enhanced with graphene. A graphene battery can be light, durable and suitable for high capacity energy storage, as well as shorten charging times. It will extend the battery’s life, which is negatively linked to the amount of carbon that is coated on the material or added to electrodes to achieve conductivity, and graphene adds conductivity without requiring the amounts of carbon that are used in conventional batteries.
Graphene can improve such battery attributes as energy density and form in various ways. Li-ion batteries (and other types of rechargeable batteries) can be enhanced by introducing graphene to the battery’s anode and capitalizing on the material’s conductivity and large surface area traits to achieve morphological optimization and performance.
It has also been discovered that creating hybrid materials can also be useful for achieving battery enhancement. A hybrid of Vanadium Oxide (VO2) and graphene, for example, can be used on Li-ion cathodes and grant quick charge and discharge as well as large charge cycle durability. In this case, VO2 offers high energy capacity but poor electrical conductivity, which can be solved by using graphene as a sort of a structural “backbone†on which to attach VO2 - creating a hybrid material that has both heightened capacity and excellent conductivity.
Another example is LFP (Lithium Iron Phosphate) batteries, that is a kind of rechargeable Li-ion battery. It has a lower energy density than other Li-ion batteries but a higher power density (an indicator of of the rate at which energy can be supplied by the battery). Enhancing LFP cathodes with graphene allowed the batteries to be lightweight, charge much faster than Li-ion batteries and have a greater capacity than conventional LFP batteries.
In addition to revolutionizing the battery market, combined use of graphene batteries and graphene supercapacitors could yield amazing results, like the noted concept of improving the electric car’s driving range and efficiency. While graphene batteries have not yet reached widespread commercialization, battery breakthroughs are being reported around the world.
Battery basics
Batteries serve as a mobile source of power, allowing electricity-operated devices to work without being directly plugged into an outlet. While many types of batteries exist, the basic concept by which they function remains similar: one or more electrochemical cells convert stored chemical energy into electrical energy. A battery is usually made of a metal or plastic casing, containing a positive terminal (an anode), a negative terminal (a cathode) and electrolytes that allow ions to move between them. A separator (a permeable polymeric membrane) creates a barrier between the anode and cathode to prevent electrical short circuits while also allowing the transport of ionic charge carriers that are needed to close the circuit during the passage of current. Finally, a collector is used to conduct the charge outside the battery, through the connected device.
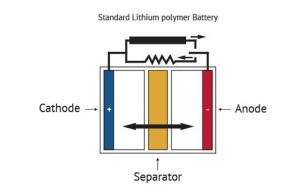
When the circuit between the two terminals is completed, the battery produces electricity through a series of reactions. The anode experiences an oxidation reaction in which two or more ions from the electrolyte combine with the anode to produce a compound, releasing electrons. At the same time, the cathode goes through a reduction reaction in which the cathode substance, ions and free electrons combine into compounds. Simply put, the anode reaction produces electrons while the reaction in the cathode absorbs them and from that process electricity is produced. The battery will continue to produce electricity until electrodes run out of necessary substance for creation of reactions.
Battery types and characteristics
Batteries are divided into two main types: primary and secondary. Primary batteries (disposable), are used once and rendered useless as the electrode materials in them irreversibly change during charging. Common examples are the zinc-carbon battery as well as the alkaline battery used in toys, flashlights and a multitude of portable devices. Secondary batteries (rechargeable), can be discharged and recharged multiple times as the original composition of the electrodes is able to regain functionality. Examples include lead-acid batteries used in vehicles and lithium-ion batteries used for portable electronics.
Batteries come in various shapes and sizes for countless different purposes. Different kinds of batteries display varied advantages and disadvantages. Nickel-Cadmium (NiCd) batteries are relatively low in energy density and are used where long life, high discharge rate and economical price are key. They can be found in video cameras and power tools, among other uses. NiCd batteries contain toxic metals and are environmentally unfriendly. Nickel-Metal hydride batteries have a higher energy density than NiCd ones, but also a shorter cycle-life. Applications include mobile phones and laptops. Lead-Acid batteries are heavy and play an important role in large power applications, where weight is not of the essence but economic price is. They are prevalent in uses like hospital equipment and emergency lighting.
Lithium-Ion (Li-ion) batteries are used where high-energy and minimal weight are important, but the technology is fragile and a protection circuit is required to assure safety. Applications include cell phones and various kinds of computers. Lithium Ion Polymer (Li-ion polymer) batteries are mostly found in mobile phones. They are lightweight and enjoy a slimmer form than that of Li-ion batteries. They are also usually safer and have longer lives. However, they seem to be less prevalent since Li-ion batteries are cheaper to manufacture and have higher energy density.
Batteries and supercapacitors
While there are certain types of batteries that are able to store a large amount of energy, they are very large, heavy and release energy slowly. Capacitors, on the other hand, are able to charge and discharge quickly but hold much less energy than a battery. The use of graphene in this area, though, presents exciting new possibilities for energy storage, with high charge and discharge rates and even economical affordability. Graphene-improved performance thereby blurs the conventional line of distinction between supercapacitors and batteries.
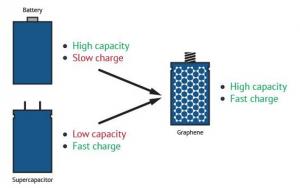 Graphene batteries combine the advantages of both batteries and supercapacitors
Graphene batteries combine the advantages of both batteries and supercapacitors
Graphene-enhanced batteries are almost here
Graphene-based batteries have exciting potential and while they are not yet fully commercially available yet, R&D is intensive and will hopefully yield results in the future. Companies all over the world (including Samsung, Huawei, and others) are developing different types of graphene-enhanced batteries, some of which are now entering the market. The main applications are in electric vehicles and mobile devices.
Some batteries use graphene in peripheral ways - not in the battery chemistry. For example in 2016, Huawei unveiled a new graphene-enhanced Li-Ion battery that uses graphene to remain functional at higher temperature (60° degrees as opposed to the existing 50° limit) and offer a double the operation time. Graphene is used in this battery for better heat dissipation - it reduces battery's operating temperature by 5 degrees.
Further reading
- Introduction to graphene
- Graphene Supercapacitors
- How to invest in the graphene revolution
- The Graphene Handbook, our very own guide to the graphene market
- Graphene-Info's graphene batteries market report
- Graphene supercapacitors market report
The latest graphene batteries news:
GMG receives regulatory development approval for battery plant
Graphene Manufacturing Group (GMG) has announced the receipt of regulatory and local council approvals for the commercial scale manufacturing of batteries at its existing Richlands site in Brisbane, Australia. To date GMG has been adhering to a research and development regulatory approval to make battery cell prototypes. In addition, this site already has council approvals that allow GMG to manufacture its graphene.
These regulatory approvals are seen an important step in GMG’s consideration at an appropriate future time to build and operate a battery manufacturing plant at the GMG Headquarters at Richlands.
Talga successfully completes USD$27 million institutional placement
Talga Group has announced the completion of an institutional placement of A$40 million (around USD$27,316,000) from the issue of 25.8 million new fully paid ordinary Talga shares. The oversubscribed Placement, managed by Euroz Hartleys Limited, was reportedly supported by a range of new and existing sophisticated, professional and institutional investors.
The proceeds will be used to fund Vittangi Anode Project early works, including earthworks and site infrastructure at the Luleå anode refinery site, commencement of procurement of anode equipment and detailed engineering, scaled up EVA production, silicon anode scale-up including securing commercial site, and general working capital (including costs of the Placement).
German chemicals specialist Evonik invests in Chinese graphene battery materials developer SuperC
German chemicals company, Evonik, has invested an undisclosed sum in China-based battery specialist SuperC.
The Chinese company produces graphene materials for, among other applications, lithium-ion batteries. The materials are said to have the potential to solve key limitations of electric vehicles and accelerate the shift to climate-friendly mobility.
Global Graphene Group's Honeycomb Battery merges with a SPAC company in a deal worth over $900 million
Global Graphene Group (G3) announced that its subsidiary Honeycomb Battery is set to merge with a SPAC company (Nubia Brand International Corp.) in a deal worth $925 million. Nubia's current valuation is $700 million, and following the merger Honeycomb Battery will become a public company that trades at the NASDAQ (ticker: NUBI), with around $118 million USD in cash.
Honeycomb Battery developed several next-generation battery technologies, which include Li-Metal batteries, Li-S batteries, silicon anodes and graphene enhanced cathode materials. The company also developed a solid-state battery technology.
GMG receives regulatory approval to enable more significant commercial sales
Graphene Manufacturing Group (GMG) has announced it has received full and final approval of all its graphene products from the Australian Industrial Chemicals Introduction Scheme (AICIS) of the Australian Government Department of Health and Aged Care under Assessment statement CA09624.
AICIS approval allows GMG to significantly increase the production and sale of GMG graphene-enhanced products including:
• Coatings: THERMAL-XR® and other industrial coatings as developed;
• Automotive Fluids: G® LUBRICANT, G® COOLANT and other automotive liquids as developed;
• Fuel: G® DIESEL ; and
• Batteries: including for GMG’s Graphene Aluminium Ion Battery.
Graphenano and the University of Valencia report major milestone with first cell made without metals
Graphenano and the Institute of Molecular Science (ICMol) of the University of Valencia have developed a battery cell without current collectors or metal terminals, which uses graphene and carbon nanomaterials instead. The system enables the creation of safer, lighter and more efficient batteries for electric cars, aviation, stationary storage and more.
The scientists have succeeded in removing the copper, aluminium or steel sheets, the materials generally used in conventional batteries to evacuate the electric current generated. Simultaneously, the metallic tabs (current terminals), usually made of nickel or other metals, which are responsible for transferring the energy from the inside to the outside of the battery, have been eliminated. The replacement of these metals by graphene and other carbon nanomaterials significantly reduces the weight and volume of the devices. Furthermore, the team reported that tests have ascertained that this replacement increases the energy density by 30-60% and eliminates the risk of explosion or fire accidents on contact with air.
Talga opens UK center for battery material technology
Talga Group has announced it has opened its new Battery Center of Excellence in Cambridge, UK. The center is a significant expansion of Talga’s UK R&D facilities, first launched in 2016.
Focusing on battery material innovations, development and characterization, the center is designed to complement Talga’s existing facilities in Sweden (Talnode®-C EV qualification production and battery
quality control labs) and Germany (processing technology scale-up and graphene production).
All-Canadian Project Arrow concept unveiled at CES, with graphene battery tech by VoltaXplore
Project Arrow, a collaboration between nearly 60 different companies in Canada that is led by the Automotive Parts Manufacturers’ Association (APMA), aims to develop an all-Canadian electric SUV. A few days ago, at the CES (Consumer Electronics Show) event in Las Vegas, a fully operational prototype was unveiled.
Reports suggest that the Canadian government contributed CAD$5 million (over USD$3.7 million) toward the electric compact SUV’s development. Ontario pledged CAD$1.8 million (over USD$1.3 million) and Quebec said it would allocate CAD$1.4 million (over USD$1 million) over 18 months to small- and medium-sized businesses that make connected or autonomous zero-emission automotive components and systems, including those looking to get involved with Project Arrow.
The Graphene Flagship's chief, Prof. Jari Kinaret, shares his views on the latest graphene development and the post-Flagship future
The Enlit Europe 2022 energy conference recently took place, and the Graphene Flagship participated, showing some of the latest energy-related graphene projects. We took the chance to discuss graphene with some of the flagship researchers, and we also talked to Prof. Jari Kinaret, the director of the flagship project, to learn of how he summarizes the last 10 years now that the flagship project will soon end.
Q: We understand that the Graphene Flagship is attending Enlit Europe 2022, showing some new graphene R&D projects. We'll be happy to get an overview of what will be displayed at the event.
At Enlit Europe, the Graphene Flagship exhibited innovations from its Spearhead Projects, which are industry-led initiatives working to move materials from research labs towards commercial applications. Among these initiatives are:
- CircuitBreakers, led by ABB and developing first-of-their-kind grease-free, maintenance-free, low-voltage circuit breakers for fault protection in key parts of the electrical grid;
- GRAPES, led by Enel Green Power and working on combining silicon solar cells with perovskite solar cells, paving the way for low-cost, highly efficient photovoltaic energy, surpassing the limits of silicon based cells.
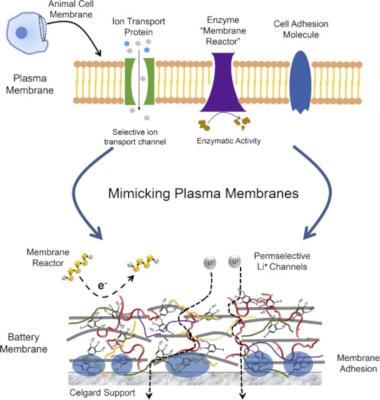
Researchers create cell plasma inspired rGO membranes for LiS batteries
Researchers from Australia's Monash University and CSIRO Manufacturing have designed a permselective membrane based on reduced graphene oxide (rGO) for making practical lithium-sulfur batteries.
The membrane closely mimics a cell plasma membrane, demonstrating selective Li+ transport and the ability to not only retain polysulfides, but also 're-activate' them on the membrane's electrochemically active interface. The team used the membrane to demonstrate high loading and high rate Li-S batteries, also on a pouch cell level.
Pagination
- Previous page
- Page 6
- Next page